Troubleshooting spa/hot tub water chemistry

By Mary Costanzo
While water quality is vital to the safety and enjoyment of both spas and swimming pools, there are also major differences when it comes to handling hot and cool water chemistry. Understanding these differences, the impact they have on water chemistry and how to manage them will make hot tub care much easier for hot tub professionals and their clients.
Spa management challenges
There are several spa management factors hot tub users and operators must contend with, including:
- extremely rapid bacteria reproduction at normal spa temperatures;
- increases in pH, due to aeration, higher temperatures and speed of flow through piping;
- less active chlorine, thanks to the tendency for higher pH values;
- more organic-nitrogen (organic-N) compounds from bathers sweat, skin oil and flakes, which react with sanitizers to create chloramines (NH2Cl);
- less water available to dilute contaminants introduced by bathers; and
- increased calcium carbonate (CaCO3) scale due to higher temperatures and increased pH.
Problems with pH
There are three primary factors working to push pH up and total alkalinity (TA) down in hot tub water.
Aeration
The bubbles introduced into the spa by the blower make the water feel comfortable and help relax bathers’ muscles. However, this action is one of several factors that strongly impact water balance for spas.
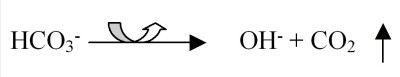
Buffering for pools and spas is based on a carbonate system. Carbon dioxide (CO2), the carbonate ion (CO32−), bicarbonate ion (HCO3−) and carbonic acid (H2CO3) work together to buffer the water against excessive swings in pH. However, the aeration (and other factors to be discussed later) causes the carbon dioxide to leave the water (see Figure 1).
The result is an increase in the hydroxide ions (OH−), which causes pH to increase. There is a loss of the carbon dioxide into the air, so the carbonate ion cannot reform, which causes a decrease in total alkalinity.
Aeration also causes more compounds within the water to sublimate into gas, which allows them to escape from the water. This affects the quantity of sanitizer present in the water, as both chlorinated and brominated compounds can be volatilized and leave the water as a gas. The air quality around the spa can also be affected thanks to the production of chloramines, which are irritating to the eyes and nasal passages.
Aeration also removes more skin cells and oil from bathers’ skin, resulting in the introduction of more contaminants than in a swimming pool environment.
Speed of circulation through piping
Spas have much faster turnover rates compared to pools. Water moves rapidly through the piping, which also has more turns than pool piping. As water flows through the elbows of the piping, the more rapidly moving water, particularly at the outside of the elbow, creates a difference in pressure that causes carbon dioxide to leave the water. Once again, the effect is an increase in pH and a decrease in total alkalinity.
Water temperature
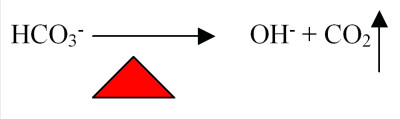
The higher operating temperatures for spas have several ways that they impact the water. Again, the tendency for carbon dioxide to leave the water is increased as the temperature of the water increases (see Figure 2).
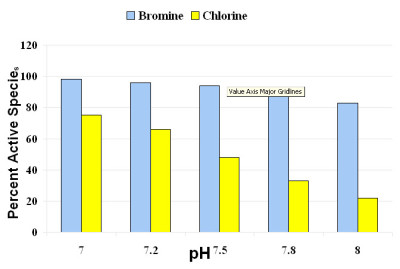
As pH is increased, there is an affect on the amount of sanitizer available to actively kill bacteria. The impact is more dramatic for chlorine than for bromine in the typical pH range for spa operation (see Figure 3).





