How does cyanuric acid affect chlorine efficiency?

Balancing cyanuric acid levels for safer pool water
By Kevin Vlietstra
Pool water needs chlorine to keep it safe for bathers. It is added primarily as a sanitizer, but it can also act as the primary oxidizer of waste and prevent algae from multiplying. When there is plenty of chlorine available, it can be used to do all three jobs. But, when it is in shorter supply, other supplements can be added to decrease the overall amount of chlorine needed, or to extend the longevity of the existing chlorine.
Cyanuric acid (CYA) is commonly used as an additive to stabilize, protect, and conserve chlorine in its tablet and liquid forms. This article will focus primarily on the role of CYA to help conserve chlorine, as well as how it affects water quality as it accumulates in the pool.
What does CYA do and how is it added to pool water?
When discussing chlorine and CYA, it is important to summarize the two types of chlorine for water treatment: chlorine with stabilizers and chlorine without stabilizers. Chlorines without stabilizers are sodium hypochlorite (liquid chlorine bleach) and calcium hypochlorite (often abbreviated as cal-hypo). Chlorines with stabilizers are usually referred to as ‘trichlor’ or ‘dichlor.’ The full names of these products are reflective of how they are created. For example, trichloroisocyanuric acid (trichlor) is created from reacting ingredients with CYA to create a granular chlorine concentrate.
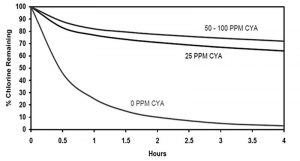
The moment dichlor or trichlor is applied to new water, CYA is introduced. The stabilized chlorine will also produce hypochlorous acid (HOCl), which will bond with the CYA. As a result, the HOCl will last longer when the pool is outdoors and ultraviolet light shines down on it. At the first part per million (ppm), chlorine is retained in the water longer than without CYA. Once the presence of CYA reaches around 25 ppm, a greater amount of chlorine (HOCl) is protected against loss in the presence of ultraviolet light.1 When chlorine stays in the water, both time and money are saved for the pool owner.
Using chlorinating compounds with stabilizer will continuously add CYA to the pool. However, once CYA levels reach 50 ppm, the benefits of chlorine retention start to level off. Looking ahead to higher levels, such as 100 ppm, there are no significant benefits to having more CYA in the water.
From a closer perspective, hypochlorite ions (OCl-) are formed with HOCl when chlorine enters the pool. When the pH is low, more HOCl is available at the time of chlorine addition. The opposite is true when the pH is higher. Another factor in HOCl availability is the presence of CYA. When CYA is present, even at the first ppm, it has an immediate negative impact on the dissociation of HOCl and OCl-. Adding more CYA will increase this impact. This is likely one of the reasons using CYA is not recommended in commercial pools.





