Inorganic chloramine reactions
As mentioned earlier, ammonia is one nitrogen atom and three hydrogen atoms (NH3). Therefore, when ammonia enters water, three types of inorganic chloramine reactions can occur:
Mono-chloramines form when the ammonia molecule gives up one hydrogen atom for one chlorine atom (NH2Cl + H2O).
- Di-chloramines form when ammonia gives up two hydrogen atoms for two chlorine atoms (NHCl2 + H2O).
- Tri-chloramines form when ammonia gives up all three hydrogen atoms for chlorine atoms (NCl3 + H2O).
- Mono-chloramines can lead to some minor eye irritation to swimmers, while di-chloramines will cause eye irritation and leave a strong chlorine odour in the air. On the other hand, tri-chloramines are very harmful. Also known as tri-halo methane or nitrogen trichloride, tri-chloramines can cause severe lung and breathing problems and are carcinogens. Nitrogen trichloride is formed when swimmers pee or perspire in the pool.
The pro-active practice of proper, regular oxidation can prevent the accumulation of detrimental chloramines. As mentioned previously, MPS, ozone, or UV systems can help to prevent the build-up of harmful chloramines.
The real problem
Ammonia does not exist in pool water due to the quick reactions that take place. Products that contain ammonia are not the problem in pools. The real problem is from organic nitrogen when it combines with chlorine to form organic chloramines. The main source of stubborn organic chlorine is from organic debris and excessive swimmer waste and not from using ammonia-based products.
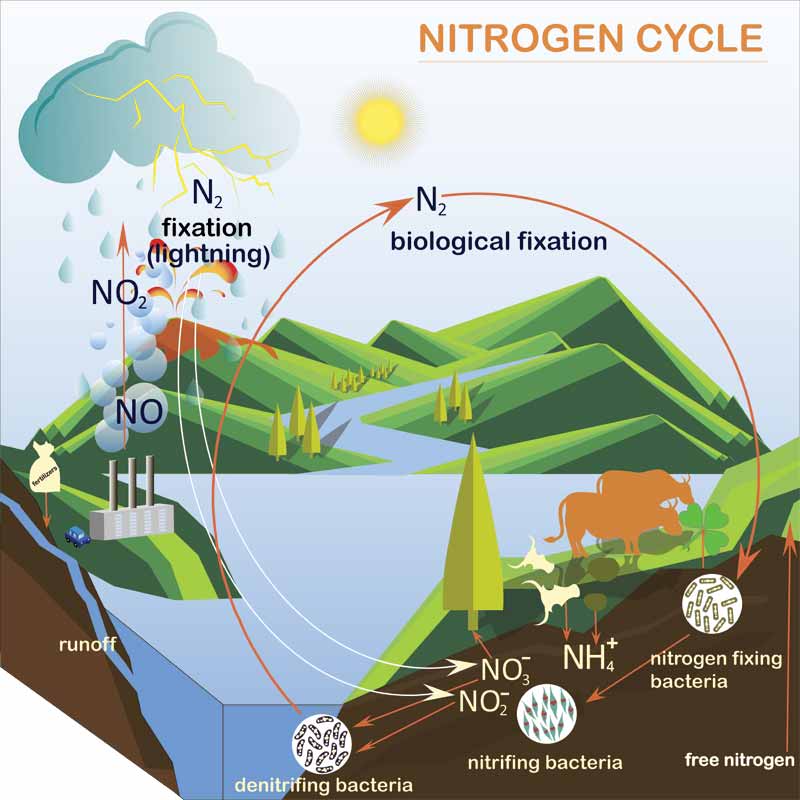
The nitrogen cycle
Nitrogen makes up 78 per cent of the atmosphere. Easily assimilated through our breathing, oxygen makes up 22 per cent of the atmosphere. If it were not for oxygen, fire would not burn and no one would be able to breath. That said, while nitrogen is more abundant it also is more difficult to distribute into the environment. Humans, as well as animals, need nitrogen for the synthesis of proteins and in the form of nitric acid for proper blood flow. Nitrates are also a primary ingredient in fertilizers for food production and is key to the production of gunpowder.
First, some history. Near the beginning of the First World War, the world was on the brink of food shortages and the availability of gunpowder was also scarce. This was until German scientist Fritz Haber discovered a method for creating ammonia by synthesizing it from atmospheric nitrogen. His process of nitrification of ammonia proved successful and there was enough nitrate derived to grow the world’s food and to fight its battles.
Fixation is the process that releases nitrogen from the atmosphere by lightning strikes or bacteria in soil. The nitrifying process starts when nitrogen in air pockets of the soil fix to bacteria. Further reaction of bacteria with nitrogen leads to ammonia. Ammonia is broken down further into nitrite and, then, finally as nitrates. In soil and certain plants, denitrifying bacteria converts nitrates back into nitrogen. The released nitrogen then returns to the atmosphere. This is the nitrogen cycle.
Nitrogen ends up in pools from storms, via human or animal waste, and algae growth. Poor disinfection, algae, or lots of organic debris will increase nitrates. Replication of the nitrogen cycle takes place in poorly maintained pools by certain nitrifying bacteria like Pseudomonas (a common bacteria found all over the world in soil, water, and plants).
Pollen can distribute nitrogen into pools, too. Excessive blue-green algae in pools contains cyanobacteria, which is nitrogen fixing. Green pools take more nitrogen from the atmosphere and nitrification leads to more nitrates. In farm areas, nitrates are present in groundwater; therefore, it is important to test for nitrates before filling a pool with well water. The recommended maximum level for nitrates in drinking water is 10 parts per million (ppm). This also applies to pools although some industry experts say 10 to 25 ppm is allowable. High levels of nitrates do not pose a health threat to adults; however, consumption of nitrates by infants and toddlers can lead to hemoglobin or ‘blue baby syndrome.’ This condition is a result of a lack of sufficient oxygen to the red blood cells.
Understanding the effects of nitrates
The existence of nitrates in pool water are what causes the majority of quality issues. Ninety per cent of ammonia in pools oxidizes to nitrogen then releases back into the atmosphere. For this reason, there can never be a residual of ammonia in pool water. The nitrogen produced by the entrance of ammonia into pools releases quickly as a gas. Heavy bather loads with excessive waste, large amounts of organic debris, storms, and algae are the primary culprits to high nitrate levels in pools.
In short, a well-maintained pool free of organic debris with routinely performed practical oxidation will not experience problems from nitrates. While storms bring in nitrates, immediate shocking afterwards can help keep them at bay. Dealing with algae quickly will also reduce their formation. Nitrate levels higher than 25 ppm can lead to rapid decomposition of free chlorine and excessive algae problems.
Removing nitrates from pool water
The most practical and cost-effective way to reduce nitrate levels in a pool is by draining and dilution. Diligence in maintenance and pro-active oxidation also helps prevent the cause of nitrates. After heavy bather loads, storms, application of fertilizers in proximity of the pool or any other unusual contamination event, the pool should be immediately oxidized. Enzymes can also be used to treat or eradicate excessive organic debris from leaves, grass, or pollen, while clarifiers can help remove nitrogen containing particulate from filters. Regular backwashing and filter cleaning will also help to keep bacteria and nitrogenous materials from forming inside the tank.
 Terry Arko has more than 40 years of experience in the pool, spa and hot tub industry, working in service, repair, retail sales, chemical manufacturing, and product development. He is a certified pool operator (CPO) instructor through the National Swimming Pool Foundation (NSPF). He also serves as instructor for the Pool Chemistry Training Institute (PCTI) to certify residential pool techs. Arko is an active member on the Association of Pool and Spa Professionals (APSP) Recreational Water Quality Committee (RWQC). He is a member of Pool & Spa Marketing‘s Editorial Advisory Committee and currently serves as a water specialist for NC Brands, parent company of SeaKlear, Natural Chemistry and Coral Seas. He can be reached via e-mail at tarko@ncbrands.com.
Terry Arko has more than 40 years of experience in the pool, spa and hot tub industry, working in service, repair, retail sales, chemical manufacturing, and product development. He is a certified pool operator (CPO) instructor through the National Swimming Pool Foundation (NSPF). He also serves as instructor for the Pool Chemistry Training Institute (PCTI) to certify residential pool techs. Arko is an active member on the Association of Pool and Spa Professionals (APSP) Recreational Water Quality Committee (RWQC). He is a member of Pool & Spa Marketing‘s Editorial Advisory Committee and currently serves as a water specialist for NC Brands, parent company of SeaKlear, Natural Chemistry and Coral Seas. He can be reached via e-mail at tarko@ncbrands.com.






