Chlorine stabilization
To slow down the natural degradation of unstabilized chlorine by the sun’s ultraviolet (UV) light, cyanuric acid is added to the water (whether supplemented separately when using inorganic chlorine or as part of the physical makeup of organic chlorine in trichlor and dichlor). Cyanuric acid is an odourless, white, granular substance with a maximum solubility of 1600 ppm in water. It is a weak acid when dissolved and will provide protection from UV light for hypochlorous acid in outdoor pools/hot tubs. An ideal concentration of 30 to 50 ppm CYA will permit HOCl to last three to five times longer under sunny conditions than unstabilized water. Cyanuric acid is not appropriate for indoor pools/hot tubs.
Currently, there is no pool chemical available proven to be effective in removing excess CYA. The accepted methods for reducing high levels are to drain and add fresh water that does not contain CYA, or use reverse osmosis systems to reduce all total dissolved solids. Splash out, carryout, or backwashing will only lower the levels slightly.
Bromine
Bromine is the other effective sanitizer and oxidizer all rolled into one product. It, too, is available in a variety of forms.
Bromochloro-dimethyl-hydantoin (BCDMH)
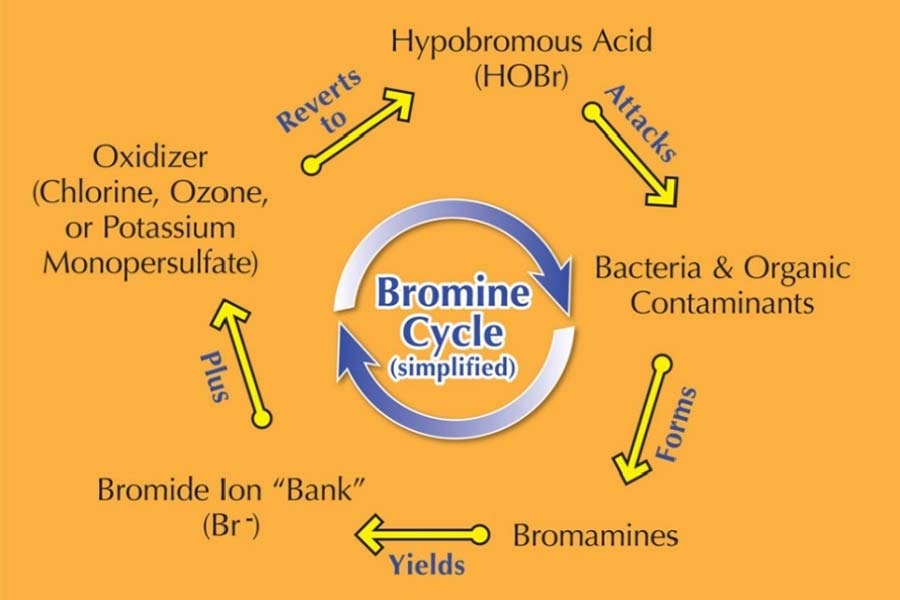 Bromine is most commonly sold as a slow-dissolving tablet used in an erosion feeder or surface floater. Also referred to as ‘organic bromine,’ BCDMH has a pH of 4.5 to 4.8. Notice the letter ‘C’ in the product, indicating BCDMH has a small amount of chlorine as part of its makeup. This is added since bromine needs an oxidizer (chlorine, ozone, potassium monopersulfate) to give it a kick-start. (see Figure 3, Bromine Cycle [simplified])
Bromine is most commonly sold as a slow-dissolving tablet used in an erosion feeder or surface floater. Also referred to as ‘organic bromine,’ BCDMH has a pH of 4.5 to 4.8. Notice the letter ‘C’ in the product, indicating BCDMH has a small amount of chlorine as part of its makeup. This is added since bromine needs an oxidizer (chlorine, ozone, potassium monopersulfate) to give it a kick-start. (see Figure 3, Bromine Cycle [simplified])
When added to water, BCDMH has the following chemical reaction:
BCDMH + H2O → HOBr + HOCl + DMH
Dibromo-dimethyl-hydantoin (DBDMH)
This form of bromine is sold as a slow-dissolving nugget or tablet to be used in an erosion feeder or surface floater. With a nearly neutral pH of 6.6, DBDMH has no chlorine content to give it a kick-start; therefore, an oxidizer must be added to an approved feeder or floater.
When added to water, DBDMH has the following chemical reaction:
DBDMH + H2O → HOBr + DMH
The bromine cycle
As mentioned earlier, bromine needs an oxidizer to generate the bromine cycle (see Figure 3, Bromine Cycle [simplified])—whether the oxidizer is part of the product itself (BCDMH) or is added separately (DBDMH).
Advantages
- no need to measure ‘bromamines’ (or combined bromine) as they are good sanitizers in their own right (about 87 per cent as effective as HOBr) with an approximate half-life of 20 minutes;
- does not breakdown in hot water environments as fast as chlorine;
- preferred sanitizer for hot tubs because it is less pH-dependent and does not dissipate quickly in hot water; and
- great for indoor environments because there is no smell or irritations associated when used properly.
Disadvantages
- has no commercially available stabilizer;
- dissipates faster in outdoor environments than stabilized chlorine;
- overdosing bromine may lower total alkalinity; and
- is a weaker oxidizer than chlorine; in hot water environments particularly, shock with chlorine or monopersulfate regularly to prevent rashes.






