Scientific studies estimate there are 30,000 to one million different species of algae in existence.1 There are also several thousands of species which have yet to be classified or named. It appears even phycologists, the people who study these organisms, disagree on the exact number of known and unknown species of algae. In pools, four main types—green, yellow, blue-green, and black—are typically identified as unwanted invaders. Since these species are omnipresent, especially in the outdoor environment, keeping them out of the pool is nearly impossible. Therefore, it is paramount for the water chemistry to be balanced, to ensure algae cannot bloom.2, 3
Chlorine and pH for algae control
One of the best algae preventers and algaecides is chlorine.4 More specifically, free chlorine (FC), and even more specifically, hypochlorous acid (HOCl). It takes just 0.05 parts per million (ppm) of HOCl to effectively prevent and inactivate algae in pool water.5 FC consists of both HOCl and hypochlorite ions (OCl-). HOCl is 99 per cent effective at killing algae, while OCl- is only one per cent effective. To ensure proper prevention and killing of algae in pool water, there must be a sufficient amount of HOCl. The pH and temperature of the water play an important role in the production of HOCl. In a pool with a pH of 7.5 and a temperature of 30 C (86 F), there is 50 per cent HOCl and 50 per cent OCl-. In this case, there would be no cyanuric acid (CYA) present, so there would be more than enough HOCl to keep algae out, at FC levels of 1 to 4 ppm. If the pH were to rise to 8.0 in this particular pool, the HOCl would go down to 24 per cent. As a result, the FC levels would need to be 3 to 4 ppm to keep algae out. Evidently, there is more algae-fighting power from chlorine at a lower pH.6
What happens when CYA is added?
If CYA is added at a level of 30 ppm, conditions change considerably, and maintaining an FC level of 1 to 4 ppm may not be sufficient to keep algae from blooming. Here is why: in a pool with 30 ppm of CYA and a pH of 7.5, there will still be 50 per cent HOCl and 50 per cent OCl- as FC. However, 97 per cent of the FC is bound to the CYA, and only three per cent of the FC is available to disinfect and control the algae. In this case, if one takes three per cent and divides it by two, they will get 1.5 per cent active HOCl and 1.5 per cent active OCl-. The following example shows the effect CYA has on chlorine’s ability to prevent algae at FC levels of 1 to 4 ppm. Ultimately, at least 0.05 ppm of HOCl is needed to prevent algae.7
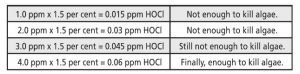
As CYA levels increase, more chlorine will be needed to create the sufficient level of HOCl required to keep algae from growing. In this scenario, pH is still important; maintaining a lower pH will help to provide a higher percentage of HOCl. With a pH of 8.0 and a CYA level of 30 ppm, there will be a 15 per cent reduction of HOCl in the water. This means the pool would have to be maintained at no less than 4 ppm of FC to keep algae out. In contrast, if the pH were lowered to 7.3, there would be more than a sufficient amount of HOCl at just 2 ppm to kill and prevent algae. Managing both pH and CYA is an important step toward keeping algae from blooming in the pool.