Mastering water’s behaviour to prevent pool chaos
By Terry Arko
The water in backyard pools is as unruly, chaotic, and unpredictable as a tempest in the open sea. In fact, it is the very nature of water itself to be outlandish and destructive. If water does not have enough food, it will eat through solid materials to get what it wants. The two favourite foods for hungry water are calcium and magnesium. These also happen to be two of the ingredients found in pool plaster.
Hungry water is “soft water.” Soft water needs minerals, and it will eat through cementitious surfaces, such as plaster, to get what it craves. When water has absorbed enough minerals, it will deposit its contents in the form of hard, crystallized calcium carbonate onto any available surface. This over-mineralized water can best be described as “hard water.” Hard water will leave its damaging contents on pool walls, floors, and especially on heat exchangers.
Water can do other unpredictable things such as invisibly carry metals into the pool, only to later have them revealed as stains upon the surface. Water is called a “universal solvent,” and while that may sound like a benign title, it means water is tirelessly working 24 hours a day, breaking down and destroying any surface capable of erosion. The challenge of every pool professional is to efficiently manage one of the most reactive and unpredictable elements on earth.
Reactive water
While water is classified as an inorganic element, it behaves in many ways like a living organism. Water is termed amphoteric, which means it can make both acids and bases. Pure water self-ionizes, maintaining a pH of seven through a process in which water molecules split and combine to form hydronium ions and hydroxide ions.
Hydronium ions are formed when H+ combines with H2O and forms H3O+. In pure water, this occurs because of a constant disassociation of water molecules, allowing for H+ to be available to attach to the strong pull of the negative oxygen charge of the H2O molecule.
This reaction also produces OH- ions. These ions are basic or alkaline, while H3O+ is an acid. Water molecules (H2O) are considered neutral in pure water and act as a buffer against the acidity of H3O+. The OH- ions also contribute to maintaining the balanced pH of pure water at seven. In pool water, H3O+ is formed when acid is added. The reactivity of pool water has to do with the immediate reactions that take place when chemicals encounter water.
When water and compounds meet

The main and most prevalent reaction involving water is known as hydrolysis. When chemicals meet water, an immediate reaction occurs, during which water breaks down the bonds present in certain compounds or substances.
The best example of this process can be observed when chlorine is added to water. The immediate reaction of Cl2 + H2O results in the formation of HOCl and HCl, both of which are acids. HCl is a strong acid, while HOCl is a weak but strong oxidizing acid. Subsequent hydrolysis leads to the formation of OH- ions, hypochlorite ions, and the byproduct of the chlorine compound. For example, adding calcium hypochlorite to water initiates hydrolysis, yielding HOCl, HCl, and OH-. The byproduct of calcium hypochlorite left behind will be calcium chloride.
All these reactions are the result of hydrolysis or water’s ability to chemically break down compounds. Water is also breaking down any substance it has continual contact with, such as plaster or any cementitious surface, contributing to the gradual erosion of solid materials.
Here are five steps that can help to ensure pools are more predictable and less chaotic:
-
Know the source water

It is important to be aware of the water composition coming from the source that will be going into the pool. Every test done on the pool should also be performed seasonally on the tap water. Both free and total chlorine levels should be tested in the source water. This is important because many water facilities now use a method of disinfection called “chloramination.” In short, this means every time water is added to the pool, the concentration of chloramines also increases. Without regular oxidation of the pool, these chloramines from the source water, along with others in the pool, can overwhelm the free chlorine, making pool sanitization more difficult.
Test pH, total alkalinity (TA), calcium hardness, and total dissolved solids (TDS). Knowing these values is important for adjusting the pool water as needed when adding makeup water or conducting a drain and refill. Two important readings from the source water are calcium hardness and TDS. When filling a new pool, the calcium hardness must be at a minimum 150 parts per million (ppm). Failure to meet this requirement may lead to issues, such as etched plaster, unless steps are taken to increase the calcium level. To illustrate, consider the tap water in Seattle, Washington, which has a calcium hardness level of 40 ppm. Using this water without testing and knowing the calcium hardness level could result in damage to the plaster.
Calcium levels can be increased by using calcium chloride according to the provided directions. The next vital test of source water is TDS. This is essential to know because when TDS in the pool water exceeds the source water by 1,500 ppm, it will result in a 50 per cent reduction in chlorine effectiveness.
Further, testing for phosphates and nitrates in the source water is recommended. If the source is from wells, it is advisable to test for metals such as iron, copper, and manganese. If any level of these metals is present, adding a good metal-sequestering product to the pool immediately upon filling is recommended.
-
Understand what chlorine is doing in the pool
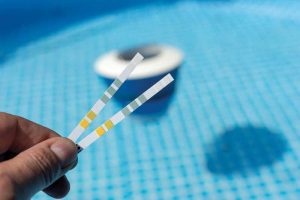
There are two main tests that should be done every week: total chlorine and free chlorine. While the focus for pools is often free chlorine, it is important to understand why testing total chlorine is necessary. When testing for total chlorine, one measures the complete quantity of any chlorine species in the pool—this means both free chlorine and combined chlorine. So, what is combined chlorine, and does it offer benefits or pose harm to the water? Another term for combined chlorine is a bit more nefarious—“chloramines” because they can cause a lot of chaos in the pool.
Chloramines in pools are formed when free chlorine reacts with waste materials in the water, such as nitrogen compounds which come from perspiration, urine, body oils, etc. This reaction leads to poor disinfection, strong irritating chlorine odours, and irritation of the eyes and skin in swimmers. So, it is not good enough to only know what the free chlorine level is without understanding the total chlorine. Testing total chlorine and understanding both the combined chlorine and free chlorine level is essential. If the level of combined chlorine is higher than the free chlorine, it can lead to chaos in the pool water.
According to the Pool and Hot Tub Alliance (PHTA), the maximum allowable level of chloramines or combined chlorine is 0.4 ppm, with the ideal target being no higher than 0.2 ppm. Regular oxidation methods, such as proactive shocking, super chlorination, ozone, UV, or advanced oxidation process (AOP), are all effective ways to minimize the formation of combined chlorine and prevent chaotic scenarios.
To determine combined chlorine, one should start by testing both total and free chlorine. Then, subtract the free chlorine from the total, and the remainder represents the combined chlorine level.
Total chlorine – free chlorine = Combined chlorine
Another critical consideration with chlorine is understanding the byproducts associated with the type being used and their impact on the water. For instance, the byproduct of trichlor is cyanuric acid (CYA). While a target CYA level of 30 to 50 ppm is ideal in the pool, excessive amounts can lead to chaos in the form of increased chlorine demand and unexpected algae outbreaks. For every 0.45 kg (1 lb) of trichlor used in 37,854 L (10,000 gal) there is 6 ppm of CYA left behind in the water. Therefore, 22.6 kg (50 lb) of trichlor used throughout a season would raise the CYA level by 300 ppm. To maintain an algae-free pool with a CYA of 300 ppm, the free chlorine residual needs to be 22.5 ppm. Further, it is to be noted, trichlor increases TDS by 16 ppm for every 0.45 kg (1 lb) of trichlor used in 37,854 L (10,000 gal).
The byproduct of calcium hypochlorite (cal-hypo) is calcium. For every 0.45 kg (1 lb) of 65 per cent cal-hypo used in 37,854 L (10,000 gal), there is an 8-ppm increase in calcium hardness. Therefore, using 22.6 kg (50 lb) of cal-hypo seasonally would raise calcium hardness by 400 ppm. Each 0.45 kg (1 lb) of cal-hypo in 37,854 L (10,000 gal) also increases TDS by 12 ppm.
Liquid chlorine does not contain CYA or calcium, so it does not contribute to increases in either. However, liquid chlorine does contribute to TDS in the form of sodium chloride. It is important to note that while liquid chlorine increases TDS, it is the least detrimental form of TDS buildup. According to ANSI/APSP/ICC-11-2019, high TDS concentrations from sodium chloride will not cause decreased sanitizer efficiency or cloudy water.
-
Water balance is the secret
Balance is truly the key to life and water chemistry, yet achieving it can be a weekly challenge. There is often confusion about how and where to maintain water balance in pools. Firstly, there are industry standards, with PHTA providing published guidelines that include minimum, maximum, and ideal ranges. While aiming for the ideal is recommended, setting more specific targets within these standards is even better. Here are some suggested targets for water balance:
- pH 7.5
- TA 90 ppm
- Calcium hardness–350 ppm (plaster pools), 250 ppm (vinyl and fibreglass swimming pools)
- CYA 50 ppm
- TDS–Maximum 1500 ppm above the source water
One of the best ways to prevent water chaos is to strive to be as close to these targets as possible. This approach ensures pools become highly predictable in their treatment week in and week out. In other words, when this system becomes routine, there is a much less chance of unpredictable occurrences, and that is the ultimate goal.
-
Add borates
Both TA and CYA act as buffers for pH, primarily preventing it from going down. Even with the TA at the proper target, the pH can still drift up, a common occurrence, especially in saltwater pools.
Service professionals often add acid to lower the pH, which may also lower the TA. Consequently, they raise the alkalinity, and the pH drifts upwards, leading to a cycle of adjustments. Interestingly, borates also act as a buffer, but they work to prevent the pH from going up. This is one reason to add borates to the pool at a target rate of 50 ppm. Here are some additional ways in which borates can help prevent chaos in the pool:
- Prevents algae growth
- Extends the life of chlorine in the pool
- Softens the water, reducing the chance of scale formation
- Enhances sparkle and clarity in the pool
The most economical form of borate to use is boric acid, requiring less and eliminating the need for additional muriatic acid.
-
Keep the water moving

Any water treatment expert will attest that moving water is healthy water. People do not drink from stagnant cesspools, but rather from water that flows swiftly. In a pool, the health of the water is completely contingent on good circulation and filtration. Without these, the pool water will experience constant chaos. No specialty chemical can replace efficient, proper circulation, and filtration. It is crucial to have good flow and clean filters capable of removing the greatest amount of micron-sized particulate matter. Consider it this way: one could take all the vitamins in the world, but if their kidneys are failing and their blood is not circulating, they will not live very long.
When it comes to filtration, it is important to understand what it truly means. If all the water in a pool passes through the filter once, is it 100 per cent filtered? The answer is no. This is known as the “turnover rate,” and it takes four turnovers of the pool water to achieve 98 per cent filtered water. Many residential pools might not even get one complete turnover in eight hours, posing a significant problem.
Determining the turnover rate of a pool involves taking the pool’s water volume and dividing it by the known flow rate of the pump in gallons per minute (gpm), and then dividing by 60, as turnover rates are measured in hours. For example, a 75,708-L (20,000-gal) pool with a pump flow rate of 25 gpm: 20,000/25/60 = 13.3 hours for just one turnover.
Hydraulics is a complex subject that should be studied by any professional caring for pools. Understanding turnover rates, flow rates, and proper filter sizing is necessary. Pools that are improperly built with substandard equipment hindering proper turnover and flow will experience more chaos than those with good flow and proper sized equipment. When considering a new account, a thorough inspection of the equipment is essential. If the equipment is undersized, old, and located far from the pool, a pool professional may want to reconsider, as the inefficiencies in equipment can lead to chaos.
With these five steps in place, one can be better equipped to ensure the pools they maintain are more predictable and rational. This translates to less chaos at the pool and in their life.
 Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years’ experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written more than 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as voting member on the board of the Recreational Water Quality Committee (RWQC). He is a Commercial Pool Operator (CPO) course instructor, a teacher of the Pool Chemistry Certified Residential course for the Pool Chemistry Training Institute (PCTI), and a member of Pool & Spa Marketing’s Editorial Advisory Committee. Arko can be reached at terryarko@hasapool.com.
Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years’ experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written more than 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as voting member on the board of the Recreational Water Quality Committee (RWQC). He is a Commercial Pool Operator (CPO) course instructor, a teacher of the Pool Chemistry Certified Residential course for the Pool Chemistry Training Institute (PCTI), and a member of Pool & Spa Marketing’s Editorial Advisory Committee. Arko can be reached at terryarko@hasapool.com.





