Recreational water testing: Tips for anticipating and removing interferences and errors
Calcium hardness
In maintaining properly balanced water, calcium hardness is one factor that should not be ignored. Water will become aggressive if calcium hardness is too low. As a result, water in this aggressive state will try to equalize itself by drawing calcium from the shell of the pool, leading to concrete pitting. If calcium hardness is too high, calcium will start to precipitate out as scale in the form of calcium carbonate.
Nearly 100 per cent of total hardness includes both magnesium and calcium hardness. The amount of magnesium in the water has no effect on a pool’s concrete shell. Therefore, the calcium hardness test is specific to calcium and is measured in ppm of calcium carbonate.
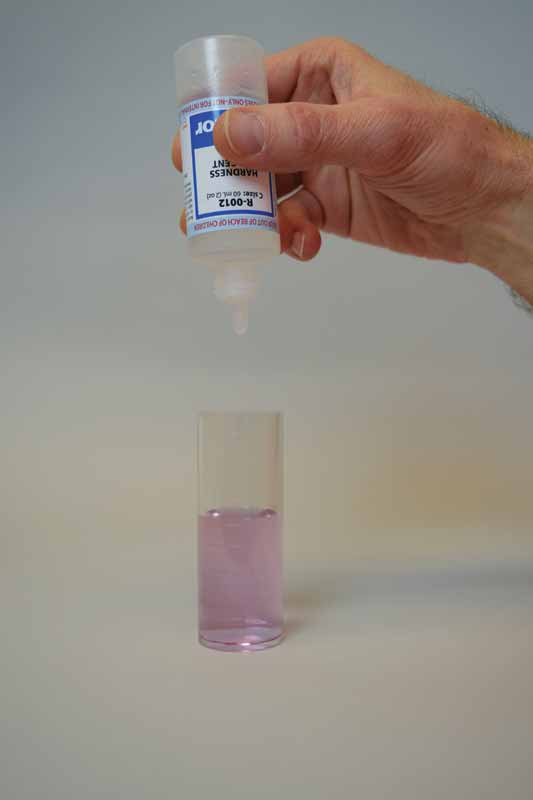
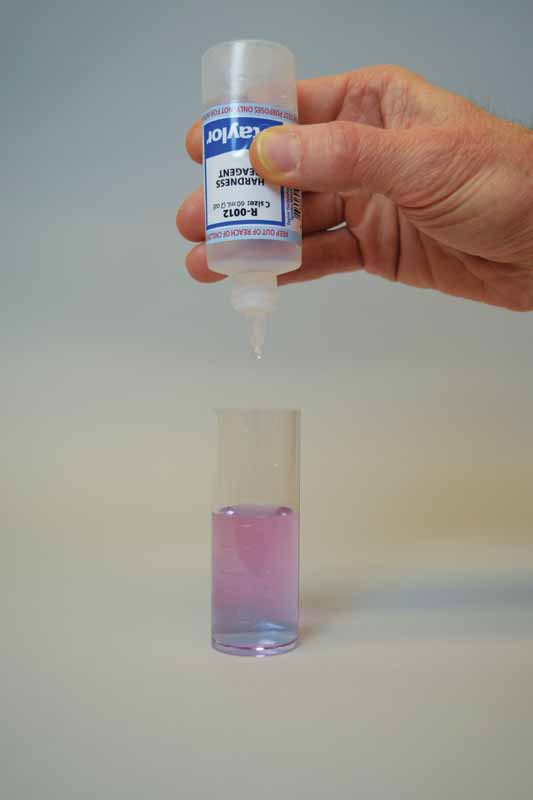
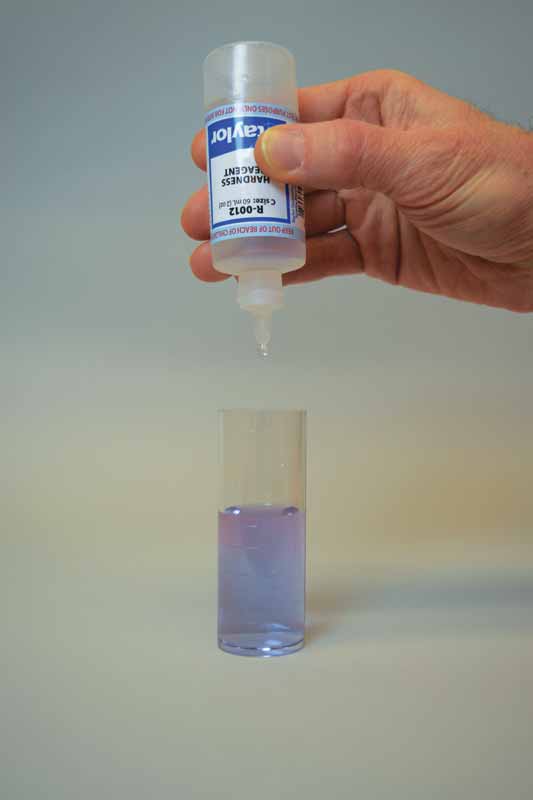
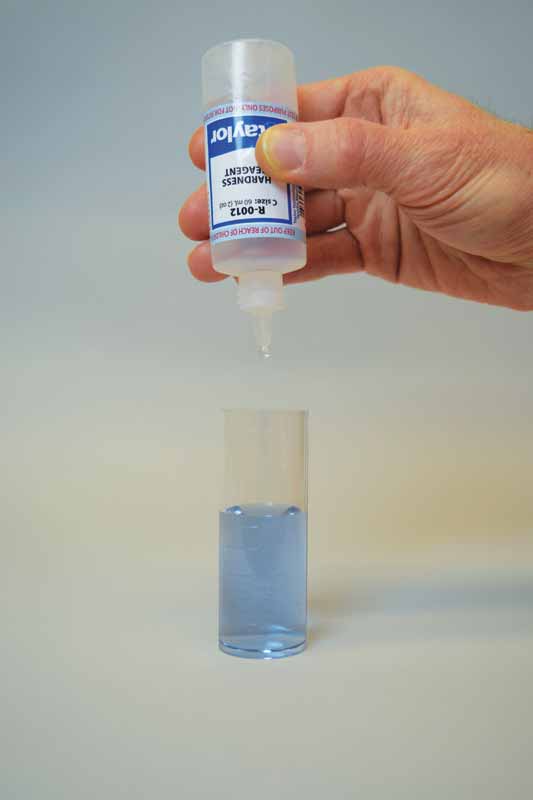
To perform this test, sodium hydroxide is first added as a buffer to increase the pH of the water sample to 10. This removes the interference of any magnesium in the sample and produces a sharper endpoint. If too much buffer is added, the calcium may precipitate as calcium carbonate and lead to a false negative result. A small amount of organic dye is then added to the buffered sample as the indicator, which changes the colour to light red.
Ethylenediaminetetraacetic acid (EDTA) is used as the titrant in this test to form a chelated soluble complex with the calcium as the EDTA binds this up. When all the calcium has been complexed, the sample will turn blue from light red. The number of drops or amount of EDTA used to achieve the endpoint is converted to ppm of calcium carbonate.
The most common interference when conducting this test involves the presence of metal ions in the sample. Metal ions interfere by causing a fading or indistinguishable endpoint. This can be rectified by adding a few drops of EDTA titrant to the sample before the addition of the buffer and indicator. The amount of EDTA added before the test should then be included in the overall amount of titrant needed to achieve the endpoint. Another solution to a fading endpoint problem is to dilute the sample by half with deionized water and then multiply the end amount of EDTA used by two.
As another cautionary measure, the titration should be conducted at, or close to, room temperature. As the temperature of the sample approaches freezing, colour change becomes very slow; inversely, if the sample is hot, the indicator can decompose.