Recreational water testing: Tips for anticipating and removing interferences and errors
Cyanuric acid
As mentioned earlier, cyanuric acid is used in outdoor swimming facilities to reduce the degradation of chlorine by ultraviolet light. The determination of pH is done by electronic or colourmetric means and total alkalinity and calcium hardness is determined by titration. Cyanuric acid uses a turbidimetric method whereby the reagent melamine reacts with the cyanuric acid to form a precipitate known as melamine cyanurate. This insoluble compound clouds the sample proportionately to the amount of cyanuric acid present.
Turbidity tests, in general, are notoriously subjective and are highly dependent upon the observer. Consider testing a standardized solution to calibrate the observer’s subjectivity.
Free and total chlorine
In determining chlorine in recreational water, the differentiation of free chlorine and total chlorine is needed. Free chlorine is the sum of hypochlorous acid and the hypochlorite ion. With pH control, hypochlorous acid, the sanitation workhorse of chlorine, can be maximized in normal swimming pool conditions. Combined chlorine is a group of compounds formed by the reaction of free chlorine with inorganic nitrogen waste and other organic contaminants. Chloramines have little disinfecting capabilities relative to free chlorine and are known to contribute to health issues with bathers. Total chlorine is the sum of both free and combined chlorine.
Due to these facts, it is necessary to know the difference between free and total chlorine. Orthotolidine (OTO), which only reacts with total chlorine, is not used in the testing of commercial recreational water. The methods used are N, N-diethyl-p-phenylenediamine (DPD) and ferrous ammonium sulphate-N, N-diethyl-p-phenylenediamine (FAS-DPD), where one is a colourmetric test and the latter a titrimetric test.
The DPD method is the colourimetric version of the chlorine test, whereby free chlorine reacts with this indicator. When this indicator is added to the sample and free chlorine is present, a reaction occurs producing the colour red. The intensity of the colour is then compared to standardized colours to determine the amount of free chlorine. Using the same sample, potassium iodide is then added; if combined chlorine is present the intensity of the red will increase. This result is then compared to the standardized colours to determine total chlorine. A subtraction calculation is used to determine combined chlorine.
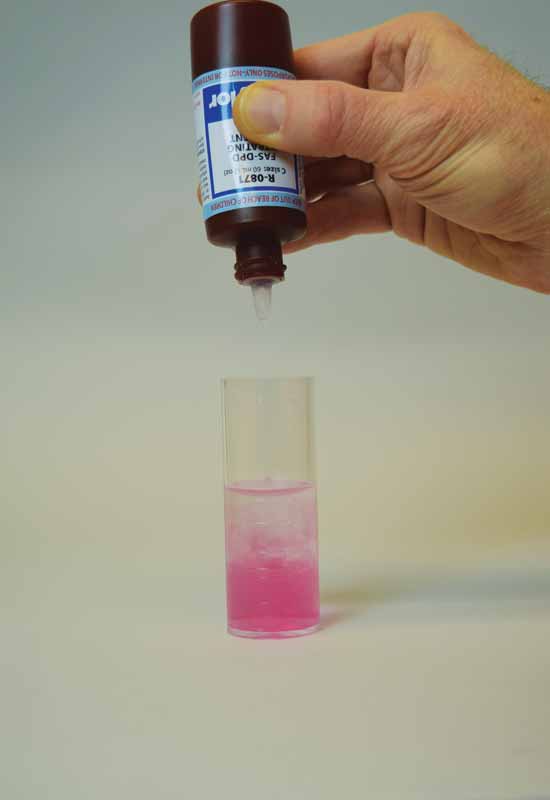
In the FAS-DPD test, DPD is used as the indicator, while FAS is used as the titrant to determine free and combined chlorine. When the DPD indicator is added to the sample—similar to the method above—the sample will turn red if free chlorine is present. The DPD indicator in this test is typically a powder and the sample size is smaller, but the same principle exists as the colourimetric DPD test.
FAS is used as a reducing agent and as the titrant is added, the original red will disappear. The endpoint occurs when the red has completely disappeared. Based on the amount of titrant added, the amount of free chlorine is determined. The subsequent potassium iodide is added, and again, if combined chlorine is present, the sample will turn red. Titrate this sample with FAS and the amount needed to eliminate the red is the endpoint, which can be recorded as combined chlorine.