
By Terry Arko
Water has long been considered one of the most vital molecules on earth. As a strong polar solvent, water can dissolve almost anything from sugar to solid rock.
Likewise, chlorine has a long history of effectively killing a wide range of dangerous germs. In fact, it has been used for well over 100 years to make the water in many communities safe, clean, and clear.
The reactive power of chlorine as a disinfectant is a result of its ability to bond with and destroy bacteria and viruses. Today, more than ever, chlorine has been called into duty during natural disasters, and against the COVID-19 virus, having widespread use both in water and on surfaces.
The amazing properties of water and chlorine work together to create reactions that lead to the destruction of a myriad of harmful germs. Water acts as an ideal carrier for chlorine in certain forms such as sodium hypochlorite. Chlorine undergoes many rapid changes in the process of oxidation, sanitization, and disinfection.
Hypochlorous acid: A powerful destroyer of germs since the beginning of humanity
The first and most immediate reaction that occurs when chlorine meets water is the formation of hypochlorous acid (HOCl) in solution with the water. HOCl has existed as a germ-killer since the beginning of humanity; it is produced by one’s white blood cells to fight off invasive bacteria that can enter the body through cuts and wounds. The existence of HOCl outside the human body was first observed by Michael Faraday in 1823 when he was experimenting with the electrolysis of brine solutions.
Faraday’s technology is what led to the manufacturing of liquid chlorinating compounds and salt chlorine generators in pools.
HOCl is the workhorse created from the addition of a chlorinating compound to destroy bacteria and prevent algae in pools. A secondary occurrence of mixing water with chlorine is the reaction from hydrolysis of the HOCl to create hypochlorite ion OCl-.
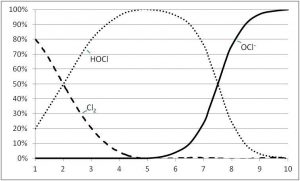
In comparison to HOCl, the OCl- is a very weak sanitizer. It represents only one per cent destructive ability of bacteria and viruses. HOCl is the strong killing agent of chlorination with 99 per cent destructive ability.
Knowing this, it is obvious that when adding chlorine to sanitize or disinfect a majority of HOCl over OCl- would be preferred. This is an important point, as the transition from HOCl to OCl- ions goes back and forth in a water system depending upon the pH. Higher pH leads to a higher percentage of OCl- and weaker disinfection. Lower pH leads to higher percentages of HOCl and greater destruction of germs in pool water.
In a pool with no cyanuric acid (CYA) and a pH of 7.5, HOCl and OCl- are both at 50 per cent. With water chemistry everything is about balance, and ideally in pools the pH is 7.5 to keep the water more neutral. If the pH is too low, the water could become corrosive; if it is too high, it could lead to scaling and staining from metals. Conversely, in a pool with no CYA and a slightly higher pH of 8, only 20 per cent of the better killing agent HOCl is available.
Cyanuric acid and chlorine
Chlorine in pool water is effective at the removal of bacteria and viruses and the prevention of algae growth. However, in sunlight HOCl is very unstable. Fifty per cent of chlorine in pool water can be destroyed by ultraviolet (UV) sunlight in less than one hour. HOCl and OCl- make up much of free chlorine (FC). UV sunlight could quickly cause a drop in the concentration of FC below the Environmental Protection Agency (EPA) recommended levels of one-to-four parts per million (ppm). This can lead to the risk of disease to swimmers.
CYA is a stabilizer; when used at proper levels, the FC in pool water will last three to 10 times longer than without it. Therefore, it is important to keep CYA at the proper concentration in the pool.
Emerging research is showing that high levels of CYA lead to a decrease in the effectiveness of HOCl to kill bacteria and prevent algae. Additional research has recently revealed the presence of CYA in pool water supersedes the effect of the pH on the percentage of HOCl to destroy germs. In other words, the CYA level in the pool water is the more prevalent determiner of HOCl levels rather than the pH. Also, it is believed that in the presence of CYA a pH of 8 will not have the same effect in reducing the HOCl.
It should be noted, however, there are many other reasons for keeping water balanced according to standards. For instance, high levels of CYA will bind to most of the free chlorine and reduce the effectiveness of HOCl to destroy bacteria, while running a pH at 8 or above will lower the oxidation reduction potential (ORP) of the HOCl as well.
ORP is a system used for measuring the oxidative potential of chemicals such as chlorine in water. It is measured in millivolts (mV). Reducing chlorine’s ability to oxidize increases the threat of disease-causing bacteria. Chlorine in pool water serves a primary purpose to sanitize by reducing the numbers of threatening micro-organisms to a safe level. Chlorine also acts as an oxidizer of organic contaminants such as those from bather waste—mainly sweat, saliva, and urine. Chlorine is primary and preferred because it can do these three things in water:
- Kill bacteria;
- Oxidize organic contaminants; and
- Leave a measurable residual in the water.
There are differing opinions; however, following standards and using balance in water management is still the best way to keep the pool water safe.






