The chemistry of chlorine in pools: Keeping water safe and clear
Types of chlorine
Service professionals and do-it-yourself pool owners have some choices when it comes to chlorinating water. For many, the type of chlorine being used may be based on anything from the makeup of regional source water, storage and safety, or cost. Liquid sodium hypochlorite (chlorine) or calcium hypochlorite (cal-hypo) may be preferred in regions with softer water.
Soft water is generally low in calcium and alkalinity, so the liquid or cal-hypo naturally add to what the water needs. In areas where calcium is high, and water is hard, trichlor tablets may be preferred. Trichlor tablets contain CYA and have a very low pH. Because they are acidic, they will lower the pH and total alkalinity.
When choosing a type of chlorine, it is important to understand the byproduct that is left behind after chlorination and how this will affect the water and type of pool surface. When it comes to cost many may reach for the bargain brand; however, that may not always be the best choice. Certain types of chlorine can lead to additional cost due to the byproducts, additional chemicals or increased water draining that may be necessary. The following are the main types of chlorine available and information on the effectiveness and byproducts of each. Chlorine is available in three categories: elemental, inorganic, and organic.
Elemental chlorine: Chlorine gas
With an available strength of 100 per cent, chlorine gas is the purest form of chlorine. It is also the most economical. That said, there are additional costs involved as elemental chlorine is by far the most hazardous form used for pool water sanitization and, as a result, has strict handling regulations.
Gas chlorine can only be applied by a technician who has been registered by the federal EPA as an applicator of chlorine. It has a pH of less than 1 and it is highly acidic. Due to the very low pH, 0.9 kg (2 lb) of soda ash is needed for every 0.45 kg (1 lb) of gas used. It is also two-and-a-half times heavier than air. Chlorine gas is very irritating to mucous membranes and can cause serious health problems if inhaled. As a result of increased regulations, handling, storage, and safety concerns gas chlorine has become much less prevalent today. However, it is the standard by which all other forms of chlorine are compared in terms of efficacy and killing power against germs.
Inorganic chlorine: Cal-hypo (65 per cent)
Cal-hypo is produced by passing chlorine gas over slaked (hydrated) lime. It is a white granular material or tablets. The most common cal-hypo contains 65 per cent available chlorine although there are other higher strengths. The pH of cal-hypo is 11.7. It is preferred in soft water regions because of its calcium content.
Although an effective shock, the drawbacks of cal-hypo use show themselves in hard water environments, where it will significantly increase the calcium hardness and, in combination with alkalinity, will tend to cloud the water. Increased calcium can lead to the formation of calcium carbonate scale as well.
Because of its inherently high pH, additional acid is needed when using cal-hypo to compensate for the rise that occurs as soon as it is added. It is also classified as an ‘extreme oxidizer;’ (class 3) it is highly volatile if it comes in contact with a broad variety of organic matter such as carbonated drinks, sweat, oil, or dust. There are newer cal-hypo tablets that are classified as class 2 oxidizers and are less reactive than the original form. When cal-hypo combines with any of these materials it can cause an explosive fire. One should never mix any other chemicals with cal-hypo.
- 57 grams (2 oz) of cal-hypo = 1 ppm chlorine in 37,855 L (10,000 gal) of pool water;
- Total dissolved solids (TDS) increase per kilogram (pound) in 37,855 L (10,000 gal) = 16 ppm;
- Calcium hardness increase per kilogram (pound) in 37,855 L (10,000 gal) = 8 ppm;
- No increase in CYA; and
- Primary byproduct is calcium chloride.
Sodium hypochlorite 12.5 per cent

Made by passing chlorine gas through a solution of caustic soda, it is the most widely used chlorinating compound in the pool industry. It is not only cost effective, but also highly efficient.
Sodium hypochlorite has available chlorine of 12.5 per cent and a pH of 13.
The most notable drawback of sodium hypochlorite is its decomposition profile. It decomposes readily over time, and that decomposition is exacerbated at high temperatures. It should be stored in a cool dark area and used more readily to prevent degradation.
It is important to note, there is no CYA or calcium byproduct from the use of liquid sodium hypochlorite. It makes for a very good shock, especially for pools using chlorine generators. It can also be used as a primary sanitizer, especially in cases where reduced CYA levels are needed. The addition of muriatic acid may be needed depending on usage.
- 296 mL (10 fl oz) of sodium hypochlorite 12.5 per cent = 1 ppm chlorine in 37,855 L (10,000 gal) of pool water;
- TDS increase per litre (gallon) in 37,855 L (10,000 gal) = 30 ppm;
- No increase in calcium hardness;
- No increase in CYA; and
- Primary byproduct is sodium chloride.
Organic chlorine
These forms are blended with CYA and are also known as stabilized chlorine because they contribute additional CYA to the pool water, which provides an inherent stabilizing effect and lessens the reduction of FC due to UV and high temperature exposure in the pool.
Trichlor

Trichloro-s-triazine-trione is made by drying and cooling the sodium salt of CYA in the presence of chlorine gas. This compound has an available chlorine percentage of 90. It is available in 25-mm (1-in.) and 76-mm (3-in.) tablets, as well as in sticks and granular formats.
The granular form is effective against black algae; however, it is not recommended for use on coloured plasters, fibreglass, or vinyl-lined pools. Trichlor has a pH of 2.9 (very acidic) and, therefore, requires diligent pH and TA testing when it is used. Additional soda ash or sodium bicarb may be needed to control decreases in pH or alkalinity.
- 42.5 grams (1.5 oz) of trichlor = 1 ppm chlorine in 37,855 L (10,000 gal) of pool water;
- TDS increase per 0.45 kg (1 lb) in 37,855 L (10,000 gal) = 16 ppm;
- No increase in calcium hardness;
- CYA increase per kilogram (pound) in 37,855 L (10,000 gal) = 6 ppm; and
- Primary byproduct cyanuric acid CYA.
Dichlor
There are two forms of dichlor available.
- Sodium dichloro-s-triazinetrione dihydrate is one form, which has 56 per cent available chlorine. A white granular material, which is lightly soluble in water, dichlor has a pH of 6 to 6.8. Because the pH is very close to neutral, this form is ideal for hot tub use.
- 68 grams (2.41 oz) of 56 per cent dichlor = 1ppm of chlorine in 37,855 L (10,000 gal) of pool water;
- TDS increase per 0.45 kg (1 lb) in 37,855 L (10,000 gal) = 12 ppm;
- No increase in calcium hardness;
- CYA increase per kilogram (pound) in 37,855 L (10,000 gal) = 6 ppm; and
- Primary byproduct is CYA.
- Sodium dichloro-s-triazinetrione anhydrous 62 per cent is different from dihydrate mainly because all the bound water molecules are removed. It has a higher AC of 62 per cent and, as a result, it is used primarily for shocking.
- 61 grams (2.15 oz) of dichlor 62 per cent = 1 ppm;
- TDS increase per 0.45 kg (1 lb) in 37,855 L (10,000 gal) = 12 ppm;
- No increase in calcium hardness; and
- CYA increase per kilogram (pound) in 37,855 L (10,000 gal) = 7 ppm.
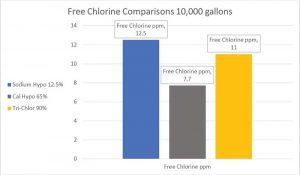
The true strength of chlorine in the pool environment
Chlorine labels can be confusing in determining the actual percentage of pure chlorine in pool water. In fact, it is a subject that would take another article to fully explore. As previously stated, chlorine gas was established as the standard in determining the amount of pure chlorine in other chlorine compounds since it is at a strength of 100 per cent. Another aspect is to compare the parts per million of FC that is provided by either a 3.8 L (1 gal) of liquid or 0.45 kg (1 lb) of dry chlorine.
In sodium hypochlorite, trade per cent is often used and is technically the volume (rather than weight) per cent of available chlorine. It is the only type of chlorine where the per cent shown on the label exactly matches the available parts per million in pool water from 3.8 L (1 gal) in 37,855 L (10,000 gal). In other words, 3.8 L (1 gal) of 12.5 per cent liquid sodium hypochlorite equals 12.5 ppm of free chlorine in 37,855 L (10,000 gal) pool water.
Dry forms of chlorine such as cal-hypo and trichlor are listed in weight per cent and not volume per cent. The available weight per cent of chlorine on the label does not match the actual percentage of free chlorine in the pool water once it is used. The chart below shows the free chlorine created in 37,855 L (10,000 gal) from 3.8 L (1 gal) of liquid 12.5 per cent compared to 0.45 kg (1 lb) of 65 per cent cal-hypo and 0.45 kg (1 lb) of 90 per cent trichlor.
Differing types of chlorine offer their advantages based on the type of facility, regional source water, availability, and ease of use. Overall, chlorine still offers the most time-proven way to inactivate disease-causing bacteria and viruses, reduce moulds and fungus, and prevent algae. It is no wonder the American Chemistry Council calls chlorine ‘The Element of Surprise’ for all the benefits it brings in keeping water clean, clear, and safe.
References
- American Chemistry Council: Chlorine the Element of Surprise https://www.chlorine.org/
- American National Standard for Water Quality in Public Pools and Spas ANSI/APSP/ICC-11 2019
- Basic Training Manual 2016 Revised Edition Independent Pool & Spa Service Association IPSSA Robert W. Lowry
- Pool and Spa Operator Handbook 2017 National Swimming Pool Foundation NSPF Pool Hot Tub Alliance PHTA
- Pool Hot Tub Alliance PHTA—APSP Fact Sheet Calcium Hypochlorite Revised June 2010 Recreational Water Quality Committee RWQC
- Pool Hot Tub Alliance PHTA—APSP Fact Sheet Cyanuric Acid Revised September 2011 Recreational Water Quality Committee RWQC
- Pool Hot Tub Alliance PHTA—PHTA Fact Sheet Sodium Hypochlorite (Liquid Chlorine) 2020 PHTA Recreational Water Quality Committee RWQC
- Pool Hot Tub Alliance PHTA—APSP Fact Sheet Trichloro-S-Triazinetrione (Trichlor)
- Sodium Hypochlorite Handbook December 2014 Oxychem

Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years’ experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written more than 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as voting member on the board of the Recreational Water Quality Committee (RWQC). He is a Commercial Pool Operator (CPO) course instructor, a teacher of the Pool Chemistry Certified Residential course for the Pool Chemistry Training Institute (PCTI), and a member of Pool & Spa Marketing’s Editorial Advisory Committee. Arko can be reached via email at terryarko@hasapool.com.





