
By France Gauvreau
Among the various industries that rely on pH meters to assist in making decisions about a process, the swimming pool and spa industry certainly represents a significant one. Therefore, routine meter calibration and maintenance are important functions that must be performed to standardize the electrode in response to its ever-changing characteristics, which are influenced by several factors such as age, temperature, coatings and chemicals.
A special bond
Before discussing the electrode’s characteristics, however, it is essential to understand the intricate bond between the electrode and the pH meter. Most people in the industry are quite familiar with pH measurement but perhaps not with pH meter operation.

Essentially, a pH meter is a highly sensitive voltmeter that uses a pH electrode to generate a millivolt (mV) potential in response to the hydrogen (H+) ion concentration in a solution (water sample). The theoretical voltage generated can be determined by the Nernst equation, which can be used in conjunction with other information to determine the equilibrium reduction potential of a half-cell in an electrochemical cell or to determine the total voltage (electromotive force) for a full electrochemical cell.
For example, at 25 C (77 F), an electrode placed in a solution with a 7.01 pH will generate zero mV and result in a ± 59.16 mV change for each pH unit from that point. A solution with a 4.01 pH, which is characterized by a higher concentration of H+ will generate a +177.48 mV (59.16 mV x 3), while a solution with a 10.01 pH and lower H+ concentration relative to 7.01 pH will generate -177.48 mV.
This is theoretical, however, and does not always represent real world behaviour. In fact, a new pH electrode will generate between +/- 10 mV in a 7.01 pH buffer solution (water mixed with a chemical to give it special properties with regards to pH. It will also have a +168.6 to +186.35 mV range with a slope percentage between 95 and 105 per cent. The slope percentage is calculated by dividing the actual voltage generated by the theoretical result and then multiplied by 100.
Electrode maintenance and solutions
Generally speaking, a well-maintained pH electrode can last up to two years. The key to longer life lies in rigorous maintenance scheduling and the use of adequate solutions. The calibration of any pH meter is also dependent on the quality of the pH buffer being used. The pH calibration process is done to standardize the glass electrode’s changes to a known reference. This basically tells the meter what voltage, which changes over time, is being generated in a solution with a known pH value.
Influential factors
There are a variety of influences on what the voltage potential will be from a pH electrode. These include the specific characteristic, at that moment in time, of the electrode and the potential for errors during the calibration process. The following are some examples of influences on electrode potential:
Hydration layer of the glass-sensing portion
A dry electrode will generate a different voltage in a 7.01 pH buffer than the same electrode that has been allowed to hydrate in a solution for a couple of hours. All glass electrodes should be stored ‘wet,’ ideally in a specific electrode storage solution to maintain the hydrated layer when not in use.
If a storage solution is not used, a 4.01 pH buffer can be utilized. However, long-term storage in a 4.01 pH buffer is not recommended as it contains phosphate. Additionally, never store a pH electrode in purified water (i.e. reverse osmosis [RO], distilled or demineralized water). Doing so will cause the electrode to prematurely fail, as the internal reference electrolyte will leach from the electrode by diffusion and cause water to enter by osmosis.
Buildup on the glass-sensing portion
A clean electrode will generate a different voltage in a 7.01 pH buffer than the same electrode that has a buildup on the glass bulb. A ‘dirty’ electrode could be disastrous to a user if a precise measurement is required.
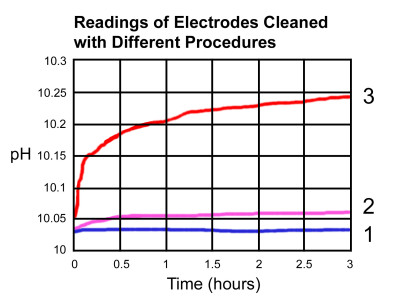 For example, this figure is from an experiment conducted with three new electrodes. Two of the electrodes were coated with a contaminant, while the third was used as a control with no coating. One coated electrode (2) was then cleaned in a specific cleaning solution. All three electrodes were calibrated to a 10.01 pH with the readings logged over three hours.
For example, this figure is from an experiment conducted with three new electrodes. Two of the electrodes were coated with a contaminant, while the third was used as a control with no coating. One coated electrode (2) was then cleaned in a specific cleaning solution. All three electrodes were calibrated to a 10.01 pH with the readings logged over three hours.
The results showed the control electrode (1) remained steady over the three-hour period, while the electrode that was cleaned (2) had a slight drift in readings, which was most likely caused by some contaminant that remained on the electrode.
The electrode that was coated and not cleaned (3) showed dramatic changes during the experiment, which was caused by the coating coming off after the calibration process. The electrode generated one particular voltage in the pH buffer while it was coated, and as it came off, it generated a different voltage in the same buffer, thus leading to erroneous results.
Physical change to the buffer
The calibration of the pH electrode is only as good as the buffer being used. Let’s say that a pH electrode generates zero mV in fresh 7.01 pH buffer and the meter is calibrated daily using a beaker containing this solution. If after a few days the voltage of the same electrode is recorded and found to be generating +25 mV, it could be possible that the voltage changed due to the electrode being dirty (as discussed above). It could also mean the buffer no longer has a pH of 7.01.
If the electrode is still working but generating +25 mV due to a contaminated buffer, then the error in measurement is going to be off by half a pH unit (theoretically, at 25 C [77 F], +25 mV would equal 6.58 pH).
Many pH meters manufactured today offer calibration check features. Based on the voltages generated by the pH electrode during the calibration process, these meters can alert the user of potential problems, including whether to clean the electrode or check the buffer. An electrode condition indicator, based on the offset (mV in 7.01 pH) and electrode slope characteristics, can also be displayed after calibration to let the user know how well the electrode is performing.
Calibration procedures
Whether or not the pH meter being used is equipped with a calibration check feature, calibration of the pH electrode has to be performed on a regular basis to ensure reliability and accuracy. If a pH electrode is new or has not been used for a while, a conditioning period is strongly advised prior to use. This should consist of storing the electrode overnight in a specific storage solution, as mentioned earlier.
A variety of pH meters are available, with each device having its own configurations and features; therefore, it is important to refer to the unit’s specifications and instruction manual before performing calibration. Some pH meters only offer one-point calibration, while more sophisticated units offer up to five-point calibration.
Additionally, various pH meters offer different resolutions—some to the pH unit (1 pH), some to the tenth of a pH unit (0.1 pH), some to the hundredth of a pH unit (0.01 pH) and some even to the thousandth of a pH unit (0.001 pH). Therefore, it is critical to select the calibration solution (buffer) appropriate for the pH meter being used.
For pool or spa applications, the most common configuration is the pH meter with two-point calibration and a resolution to the hundredth of a pH unit.
Performing a two-point calibration

Based on the above, the following is the procedure for a two-point calibration performed with a pH meter that has a 0.01 pH resolution.
- Dip the already conditioned glass bulb of the pH electrode into a 7.01 pH buffer and wait for the reading to stabilize. If the pH meter offers manual calibration, adjust it to read 7.01 on the display. If the pH meter offers automatic calibration, wait for the meter to automatically adjust itself. Then, rinse the pH electrode with distilled or tap water.
- Dip the pH electrode into a 4.01 or 10.01 pH buffer and wait for the reading to stabilize. Again, if the pH meter allows for manual calibration, adjust to read accordingly or wait for the meter to automatically adjust itself. Once calibration is finished, the meter will automatically return to its reading mode. Calibration is done and the meter is ready to be used.
 It is important to always start calibrations with a 7.01 pH buffer and then proceed with the 4.01 or 10.01 pH buffer. The choice of the second buffer depends on the application. For example, when measuring values below 7.01 pH, a second buffer of 4.01 pH should be used. Similarly, when measuring values above 7.01 pH, a second buffer of 10.01 pH should be used.
It is important to always start calibrations with a 7.01 pH buffer and then proceed with the 4.01 or 10.01 pH buffer. The choice of the second buffer depends on the application. For example, when measuring values below 7.01 pH, a second buffer of 4.01 pH should be used. Similarly, when measuring values above 7.01 pH, a second buffer of 10.01 pH should be used.
How often should the pH meter be calibrated?
Frequency of calibration is one of the most popular questions asked. Furthermore, it also generates the question of how accurate should the meter be? As no application is the same, the following guidelines can be used to determine when calibration is required.
- Start by calibrating the meter using fresh pH buffers as described above. On the following day, simply soak the electrode in the original buffers used for calibration and note the readings;
- If the readings are still within expectations, continue this procedure everyday until the meter’s accuracy is no longer sufficient. Then, proceed to calibrate the meter and note the number of days that passed since it was first calibrated. For example, if five days went by, proceed to calibrate the meter every four to five days; and
- As the meter’s pH electrode gets older, perform this test monthly to confirm the number of days required between calibrations. This is also a good way to forecast electrode cleaning or replacement
Verification procedures
If an electrode seems to drift or has a slower response time between calibrations there could be several problems, some of which are difficult to detect with the electrode. It is therefore imperative to proceed with proper verification.
First look for scratches on the sensor (glass bulb). If the electrode seems to be intact, make sure the pH electrode is well conditioned by soaking the sensor overnight in a specific storage solution.

When conditioning is completed, dip the electrode into a 7.01 pH buffer and wait for the reading to stabilize (if it does not stabilize, perform a cleaning procedure). Adjust the meter to read 7.01 pH. If the value can not be reached (reading higher than 8.20 or lower than 5.80 pH), perform a cleaning procedure.
When the meter can be adjusted to 7.01 pH, switch to mV readings (available on most pH meters). The meter should read 0.0 mV, ± 30 mV. If the reading is higher than ± 50 mV, the electrode needs to be replaced. If the reading is below ± 50 mV, proceed to rinse the electrode with distilled or tap water and dip into a 4.01 or 10.01 pH buffer.
At this point, switch the meter to pH measurement mode and wait for the reading to stabilize. Adjust the meter to read 4.01 pH. If the value can not be reached (reading higher than 5.00 or lower than 3.00 pH), proceed by cleaning the electrode with an appropriate solution.
When the meter can be adjusted to 4.01 pH, switch to mV readings. The meter should read 180 mV, ± 30 mV. If the reading is higher than ± 50 mV, the electrode needs to be replaced.
Maintenance and troubleshooting should be routine
For the conscientious user, electrode maintenance and troubleshooting is a routine part of the measurement process. A properly maintained electrode can be expected to provide consistent readings for a long period of time. The challenge, however, is realizing when usage, aggressive measurement environments or improper cleaning starts to negatively affect pH measurement.
Users should therefore take care in matching the appropriate cleaning solution to the type of sample being measured, using fresh buffers (reuse as seldom as possible) in addition to storing (conditioning) the electrode in an adequate solution when not in use. Always keep in mind that compromised measurements may be close enough to the expected results to seem plausible!
 France Gauvreau is the general manager for Hanna Instruments Canada Inc., and has been part of the Hanna team since 2000. She has worked in water analysis for the past 22 years and authors a quarterly column on water analysis instrumentation for Source Magazine. She has also contributed an article to Piscines & Spas (Fall 2010) on water testing devices. She can be reached via e-mail at franceg@hannacan.com.
France Gauvreau is the general manager for Hanna Instruments Canada Inc., and has been part of the Hanna team since 2000. She has worked in water analysis for the past 22 years and authors a quarterly column on water analysis instrumentation for Source Magazine. She has also contributed an article to Piscines & Spas (Fall 2010) on water testing devices. She can be reached via e-mail at franceg@hannacan.com.







Good day
So what is a good storage solution? We are experiencing a red build up on meters and glass. Currently we go through 15 pens every year now I believe it is because we store in 4.0. Also the rooms in which they are in is always at 85, is that another problem? Thank you