Secondary testing can keep metal content under control

By Joe Sweazy
How do pool professionals deal with cloudy, discoloured water? How about surface stains and rusty metal parts or complaints from swimmers whose hair is turning green, even when the water seems to be properly balanced. These and other symptoms can drive pool managers crazy—and they can all be a result of elevated levels of common metals in swimming pool water.
Where do metals come from?
Copper and iron are the two most common problem metals for swimming pools and spas. While they are essential micronutrients in every adult’s diet, we try to reduce or eliminate these unwanted metals in swimming pool (and, occasionally, spa water).
Source water
The most common origin of copper and iron in pools and spas is the source water itself. Water percolating through soil and rock can dissolve minerals containing copper or iron; this contaminated water eventually makes its way into wells or other reserves.
Pipes and plumbing
Municipal water supplies typically have lower levels of unwanted metals, but even if well water isn’t used to top off the pool, metals can still make their way into the water, often via old, dissolving pipes and plumbing. This and other metal equipment in your pool can corrode, particularly if the water’s pH or calcium hardness level is too low.
Chemicals and systems
Some algaecides used for pool maintenance contain copper as an active ingredient. Also, copper is commonly used in increasingly popular mineral purification systems for pools and hot tubs; the metal often plays a part in these systems because of its sanitizing abilities. As such, if a mineral purification system is being used, copper levels must be kept low (at or below 0.2 parts per million [ppm]). Otherwise, it can form scale deposits or stains.
What do metals do to the pool?
Once metals have found their way into a pool, several water chemistry factors can contribute to discolouration and scaling. High total alkalinity (above 180 ppm) can promote stains and scale formation from metals; a pH above 7.8 can also cause these problems. In addition, oxidation can occur, drawing metals out of the solution and ultimately creating worse problems.
Explaining oxidation
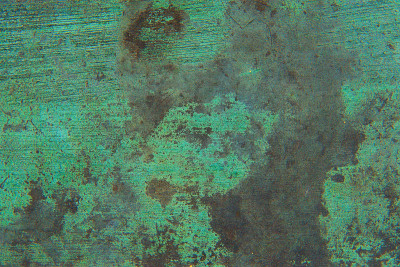
When a metal compound is exposed to air, it can combine with oxygen, creating a new form of the metal. In deep wells or piping systems, where the oxygen content is low, the water containing copper and iron is clear and colourless. Therefore, the water from the tap containing these metals not seem suspect. However, when exposed to air for some time, the metal content can oxidize into a colourful, solid form.
For example, oxidation changes iron in the water into a yellow or reddish-brown solid, while copper tends to take on a blue or blue-green shade. When these metals settle out of water in their solid form, they cause stains and rust on pool surfaces, fixtures and equipment. Smaller particles remain suspended in the water, causing a cloudy, discoloured appearance that is less than appealing to potential swimmers.

Exposure to air is just one method by which oxidation can occur. Another more powerful oxidizer is often responsible for faster metal precipitation—the free chlorine used to sanitize swimming pools. The qualities that make chlorine great for destroying unwanted debris and contaminants can also set metal staining and scale formation in motion.
The free chlorine and available oxygen in your pool will speed up the rate at which elevated levels of copper and iron oxidize. Water that looked clear and perfect from the tap then becomes cloudy and discoloured with a primary shock dosage. Even if already-chlorinated pool water is simply topped off with fresh water containing metals, there is potential for a metal problem.
This is not just limited to chlorine oxidizer. Other sanitizers or shock agents may also help oxidize metals into forms that cause stains and scale buildup.





