UV and ozone: Companions in managing pool water contamination
Synergy of UV and ozone for disinfection and oxidation
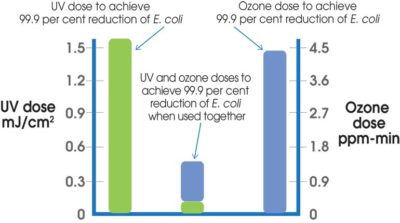
In 2006, Magbanua and co-workers clearly and systematically delineated the synergistic effect of a UV/ozone combination against E. coli.. In separate independent tests, the researchers studied the efficacy of UV and, then, the efficacy of ozone against E. coli. In doing so, the team was able to establish the requisite dosages of these two disinfectants to affect a specific level of microbial reduction.
In a second series of tests, the team began to use UV and ozone together to determine if the resulting microbial reduction was simply the sum of the contributions from each disinfectant. The results were nothing short of astounding (see Figure 4).
It was discovered the individual UV and ozone doses required to destroy E. coli to the same extent (e.g. 99.9 per cent), could be reduced by a factor of 18 and four, respectively, when the two disinfectants were used together. In essence, there was a synergistic effect on the microbial reduction of E. coli when UV and ozone were paired together in a dual disinfection strategy.
According to Magbanua et al, the synergy associated with UV/ozone water treatment is attributed to the presence of supplementary hydroxyl radicals. Hydroxyl radicals are extremely fast-reacting, potent, non-selective chemical species. In fact, their oxidation power is recognized as being far more potent than chlorine gas, hypochlorous acid (HClO), or ozone. Further, the reactivity of hydroxyl radicals has long been recognized as being extremely fast—in some instances as much as one million times faster acting than ozone for bond breaking via chemical oxidation. For these reasons, the inactivation rate of waterborne pathogens is much greater due to the additional oxidizing power provided by the supplementary hydroxyl radicals.
The benefits of pairing UV with ozone does not stop with disinfection performance. While UV has virtually no oxidizing ability, the resulting hydroxyl radicals created from UV and ozone are tremendous oxidizers. Again, as is the case with disinfection, the literature is studies that reflect the superior oxidation performance of hydroxyl radicals formed from UV and ozone.
Table 5 provides a list of some of the reported research on advanced oxidation in water treatment and the applications in which it has been employed. It is clear the breadth of the applications for the trio of technologies within water treatment is vast owing to the potency of the combination for disinfection as well as oxidation. In recognition of the tremendous advantages that a combination UV and ozone system brings to recreational water treatment, devices are beginning to appear in the pool industry which deliver these benefits.
Table 5: Research related to advanced oxidation |
|
|---|---|
| Investigation/Application | Research Findings |
| Disinfection of E. coli | Synergistic disinfection performance against E. coli using UV/O3 combination. |
| Oxidation of disinfection byproducts | Total trihalomethanes (THMs) and total organic halides reduced by 90 per cent and 98 per cent, respectively using UV/O3 combination. |
| Oxidation of organic carbon and disinfection byproducts | Total organic carbon reduced by 50 per cent over O3 or UV alone. THMs and haloacetic acids (HAAs) reduced by 80 and 70 per cent, respectively. |
| Drinking water treatment | Overall, the combination of ozonation and UV treatment leads to an important water quality with regards to disinfection, oxidation of micropollutants (atrazine, MTBE, 17-a-ethinylestradiol) and minimization of bromates. |
| Disinfection of poultry feed water | Fifty-fold improvement in disinfection against E.coli |
| Treatment of surface waters | Complete removal of MB (methylisoborneol) and geosmin and 40 per cent reduction in bromates with UV/O3. |
| Removal of disinfection byproducts | Increased reaction rate for the destruction of HAAs. |
Conclusion
It should come as no surprise that using UV and ozone (or a combination of the two) is gaining attention as a strategy for managing pool water contamination. Both contribute significantly to reducing the organic and inorganic contaminants that enter the pool from the source water, bathers, and the environment. In their role as supplementary oxidizers and sanitizers, UV and/or ozone help reduce the demand on the chlorine such that overall chlorine use is lowered. In addition to the benefit of reducing chlorine use, the pool water is more pure and healthy for bathers, a result that by any measure is a positive step for the pool industry.
 Ray Denkewicz is the global product manager for sanitization and chemical automation for Hayward Pool Products. He has worked in the pool industry for more than 15 years, including a role as vice-president of technology for Zodiac Pool Care. Denkewicz has a degree in chemical engineering and has been instrumental in developing a number of products for the pool industry, many of which are backed by more than 100 of his worldwide patents. He can be reached via e-mail at rdenkewicz@hayward.com.
Ray Denkewicz is the global product manager for sanitization and chemical automation for Hayward Pool Products. He has worked in the pool industry for more than 15 years, including a role as vice-president of technology for Zodiac Pool Care. Denkewicz has a degree in chemical engineering and has been instrumental in developing a number of products for the pool industry, many of which are backed by more than 100 of his worldwide patents. He can be reached via e-mail at rdenkewicz@hayward.com.





